Why have they become so popular?
Lithium-Ion batteries have recently become so prevalent as they are versatile, have high energy density, high capacity, and long-life rechargeable batteries. They can also work in a lot of various environmental conditions, which makes them more robust all around. The current uptake in those batteries is mainly due to falling prices, increasing customer confidence, and the continuous development of high-capacity battery technology – led by the electric vehicle industry. They are also sometimes linked to the usage of renewable energy resources, such as the case of solar powered photovoltaic cells. As they have become so prevalent, awareness of their fire hazards has also increased. Unfortunately, fire incidents have assisted in that increased awareness.
Understanding the battery itself
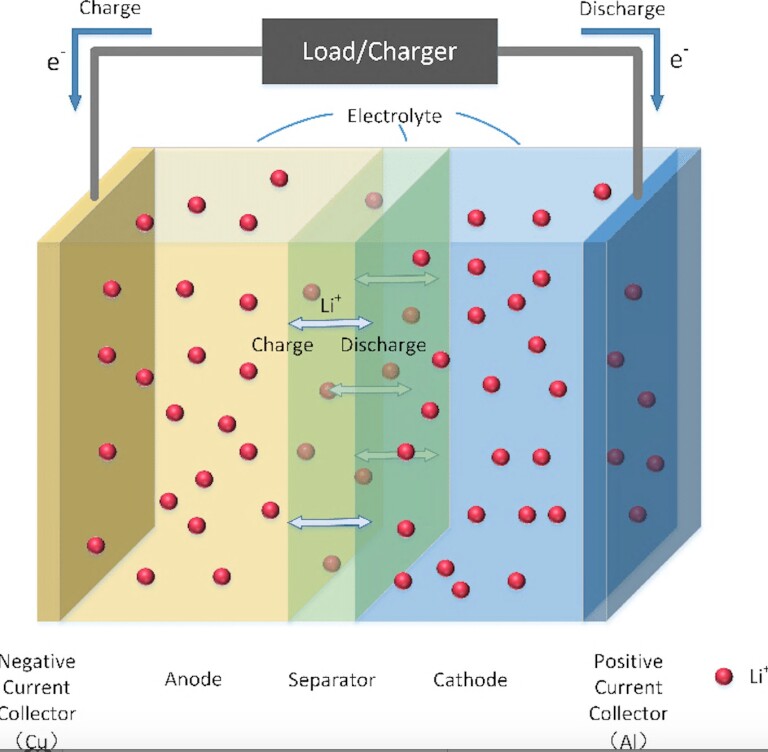
To understand the fire hazards, we’d need to look at the components of the battery itself. The main cell is composed of two electrodes, a negatively charged anode and a positively charged cathode. These are separated by a membrane that electrically isolates the two electrodes. All of this is submerged in non-aqueous electrolyte which enables the flow of the lithium ions from one side of the cell to the other.
When charging, the ions flow from the cathode to the anode and vice versa when discharging. Several types of lithium-ion batteries exist and are based on the variety of cathodes, anodes, separators, and electrolytes used. This affects the stability, energy density, usage, etc. The type of electrolyte used is a major fire risk in case of a battery failure, so it’s important to watch out for.
Lithium-ion batteries are composed of scalable modular systems. The battery system can be sized to meet specific capacities and voltages. Cells are connected in parallel to form batteries, batteries are connected in series to form modules, and modules are combined to form packs.
Fire and explosion hazards
The special fire risk formed by such batteries is thermal runway, which is defined as the uncontrollable and exponential increase in temperature due to exothermic reactions triggering additional exothermic conditions.
In other words, the battery keeps on generating heat which results in even more heat generation, which poses a major challenge for fire fighters trying to extinguish the fire and stopping it from starting again. An example of that can be seen in the video below, in which the fire service had to keep a car fully submerged in water for 24 hours to make sure that the battery was fully cooled off.
Link to Facebook post:
https://www.facebook.com/443255665688195/posts/2902012643145806/
How does thermal runaway happen?
Thermal runaway can occur via a variety of factors, be it physical, electrical, or thermal abuse. An example of that if a battery gets heated because of external sources, the components of the cell begin to break down and react with one another, this increases the temperature which triggers additional reactions. This leads to a positive feedback loop of further increasing temperatures. As this process happens, the cell contents are vaporized, and the cell pressure increases which can potentially ignite the cell itself.
As the cells are densely and compactly placed within the system, the failure of a single cell can lead to a thermal runaway involving a whole battery pack. The battery chemistry highly affects the thermal runaway and how it propagates. Nevertheless, there is a lot of heat involved in thermal runaway, so it’s not just about extinguishing a flaming combustion, but rather about removing the heat from the system so that the battery does not re-ignite, which can happen several days after.
Off-gassing and combustion
The gas composition vented depends on the chemistry of the cell itself. Off-gasses primarily include hydrogen, carbon monoxide, and carbon dioxide, as well as small quantities of carbon. During a flaming combustion, some battery types can produce hydrogen fluoride which is a highly corrosive and extremely toxic acid. Also, if the gases do not ignite and accumulate in an area, there is a significant explosion risk especially to fire fighters trying to respond to a gas leak from batteries. Peak heat release rate can also vary significantly with the battery chemistry.
Main Risk mitigation measures – Ventilation and suppression
Ventilation and suppression are the two key mitigation measures. During the early stages of thermal runaway, toxic and explosive gases are vented from the cells and if the battery ignites there will be significant volumes of smoke to deal with as well.
An appropriate ventilation system of smoke, flammable, and toxic gases is needed to continuously operate and exhaust directly to outside. This can also be achieved via an emergency exhaust system that operates promptly as soon as those gases are detected. Recirculation systems mist be designed carefully to avoid the transport of toxic/ flammable vapours and smoke. As for suppression, and even without a visible flame, a thermal runaway can still propagate within a battery system and will continue to vent flammable gases with the risk of ignition.
Thus, the suppression of lithium-ion batteries needs to consider the removal of heat from the system as reignition can occur if the temperature is not lowered enough. This renders water as the favoured suppression system, though the unwanted water damage risk can deter lots of business owners from opting for it.
Water has high heat removal capacity and is cheap and abundant. Sprinkler systems can provide some cooling and potentially reduce the fire spread but might have issues with reaching the hidden areas in the battery racks. However, direct application of water in large volumes to eliminate the produced heat has proven to be more effective. This usually requires fire fighters’ interference.
Other risk mitigation measures
Other mitigation measures can include battery management systems, state of charge, detection systems, and compartmentation and physical segregation. A successful battery management system maintains the battery within the designed operating range, as well as reporting any issues within the battery, including its temperature. They can also cut the current off and raise alarms if needed.
The state of charge is important as thermal stability, peak heat release rates, and thermal runaway propagation are usually higher with lower state of charge. As for detection systems, multi gas sensors can be used to detect combinations of various gases or volatile organic compounds. When integrated with the alarm, ventilation, and suppression systems, this can be very effective in early detection and warning. Last but not least, physical segregation and compartmentation can limit the fire growth, spread, and damage. Combined with localised ventilation and suppression strategies, damage can be contained and risk to life and business continuity can be as minimal as possible.
To wrap it up, Lithium- Ion batteries are here to stay for all their massive technological advancements and possibilities. Thus, it is up to scientists and researchers to come up with better ways to tackle their challenges and hazards while reaping their endless benefits.

